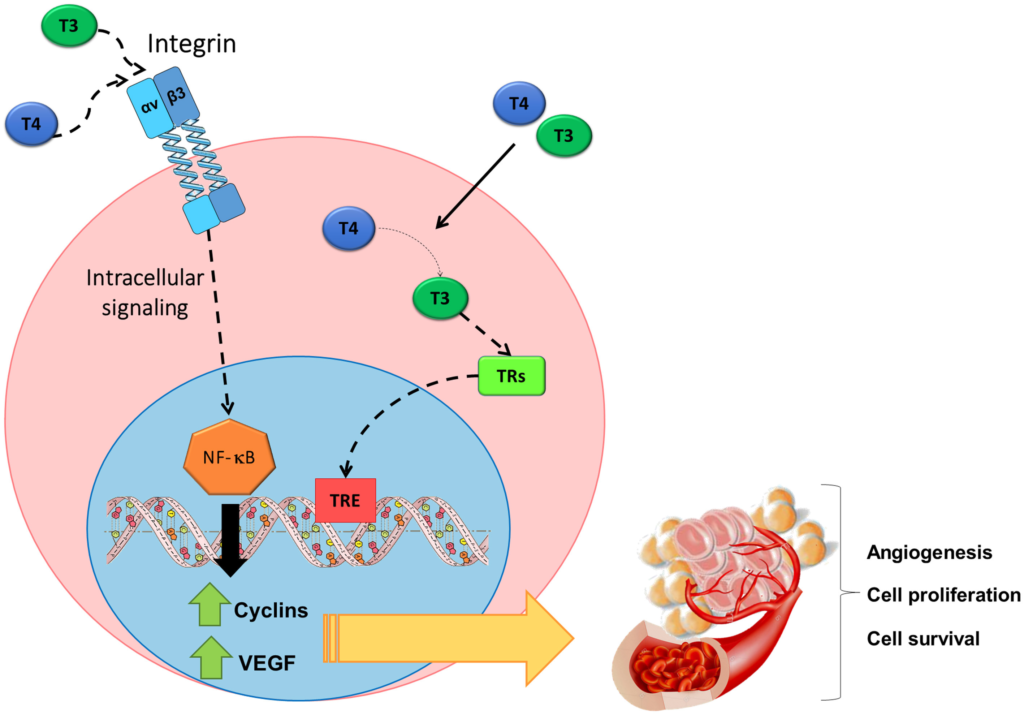
The Integrin αvβ3 Receptor and Thyroxine
The following diagram illustrates integrin αvβ3 receptors. In general thyroid hormone action is ‘genomic’, T3 binds to nuclear receptors which (along with cofactors) bind to Thyroid Response Elements (TREs) on the DNA. T4 has little ability to bind nuclear receptors, it must be converted to T3 and so T4 is considered a prohormone.
There are other, non-genomic receptors such as the Integrin αvβ3 Receptor on the cell membrane. Integrin αvβ3 binds both T3 and T4 but T4 has the dominant action. T4 is the active hormone for the Integrin αvβ3 receptor.
Figure 2 from: Cayrol, F, Sterle, HA, Díaz Flaqué, MC, Barreiro Arcos, ML, Cremaschi, GA. Non-genomic actions of thyroid hormones regulate the growth and angiogenesis of T cell lymphomas. Front Endocrinol. 2019;10:63. doi: 10.3389/fendo.2019.00063
Tetrac (Tetraiodothyroacetic Acid)
A number of studies mention Tetrac, so it is useful at this stage to know a little about it. Tetrac (tetraiodothyroacetic acid) is a deaminated analogue of L-thyroxine, as can be seen in the diagram below. Tetrac (e, in blue) is very similar to thyroxine (a, in red). Tetrac blocks the binding of T4 to the Integrin αvβ3 Receptor and is used in experiments as a potential cancer treatment. This is all we need to know about Tetrac, it stops T4 binding to the Integrin αvβ3 Receptor.
T4 binding to the integrin αvβ3 receptor increases cancer risk and mortality. The studies are in chronological order so we can see how the science has advanced in the last few years.
Cancerous Actions of Integrin αvβ3 Receptor
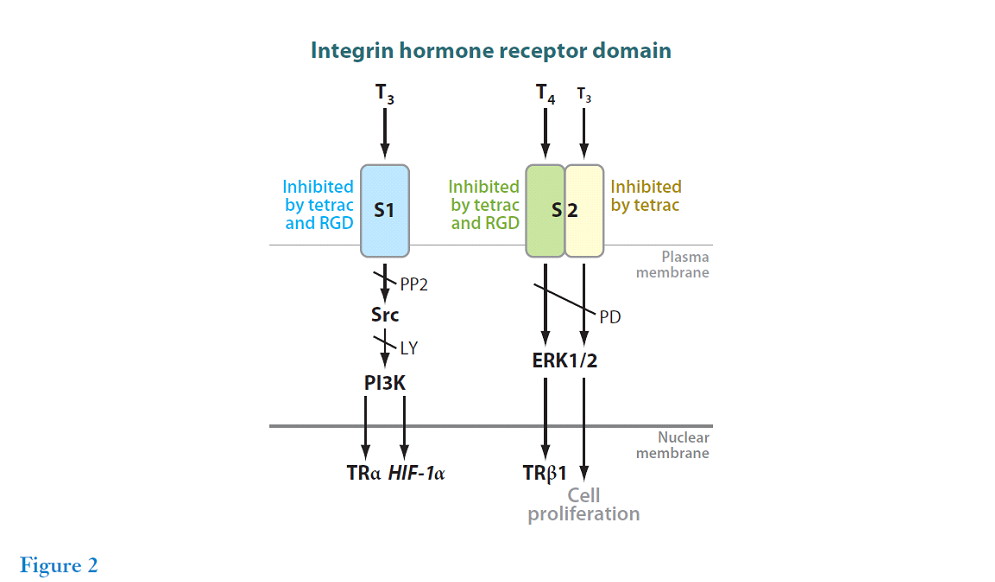
This review summarises the state of knowledge as of 2011. T4 is biologically active at the integrin receptor. The S2 pathway binds T4 and, to a lesser extent, T3 which results in cancer cell proliferation. The integrin αvβ3 receptor is widely expressed in cancer cells.
As stated earlier tetrac blocks the binding of T4 to the integrin receptor. The review describes a study in which human cancer cells were grafted into mice. “Tetrac and nanoparticulate tetrac (the latter at one-tenth the dose of unmodified tetrac) strongly inhibited the growth of human renal carcinoma cells (33), medullary carcinoma of the thyroid (34), follicular thyroid cancer cells (35), chemoresistant breast cancer cells (30, 36), and pancreatic cancer cells (37).“
The review also describes how tetrac can sensitize tumour cells to radiotherapy by inhibiting integrin αvβ3 receptor binding. “Integrin αvβ3 has been reported to modulate cancer cell responses to radiation (43). Hercbergs et al. (44) have recently shown that mouse glioma cells exposed briefly to micromolar concentrations of tetrac in vitro exhibit up to a threefold increase in radiosensitivity.“
Thus, the integrin αvβ3 receptor has a crucial role in cancer progression. We now show evidence that T4 activates this receptor (‘Hypothyroxinemia’ is a formal term for low T4).

This study looked at 23 patients with end stage cancer. They were given methimazole to suppress the thyroid and liothyronine to restore clinical euthyroidism: their T4 was replaced with T3. The study was not placebo controlled but had striking results both in terms of survival and radiologic improvement. We should not underestimate the power of the mind in such extreme circumstances. Here are quotations from the introduction to the study that illustrate the benefit of reducing T4 levels.
In a phase II clinical trial of recurrent glioblastoma multiforme (GBM) conducted by the author (A.H.), medically induced hypothyroidism was associated with significantly longer progression-free survival and overall survival rates [5, 6]. In that study, it was found that prolongation of survival correlated significantly and independently with circulating free thyroxine (FT4) levels
The integrin is amply expressed by cancer cells and rapidly dividing endothelial cells and is not well expressed by or activated in quiescent, nonmalignant cells [3]
Within the cell, T4 serves as a prohormone for T3, and T3 is the metabolically and genomically important form of thyroid hormone [1]. At the integrin, in contrast, the thyroid hormone receptor affinity for T4 is higher than that for T3 [7], and T4 is a more potent inducer of tumor cell proliferation than is T3 [9]
Addition of T3 rapidly reduced serum thyrotropin (TSH) and FT4 levels and was associated with rapid clinical and radiologic improvement [11]
Comments from the Results and Discussion of the study: –
The odds of surviving at least 12 months for 19 of 23 individual patients were estimated to be lower than 15%–20%, whereas 19 of 23 from the managed group (83%) survived more than 12 months and 12 of 23 (52%) survived more than 24 months versus an estimated 1 of 23 (4.4%).
Radiologically documented tumor regression was observed following exogenous l-T4 withdrawal alone (n = 3). These were unusually rapid and durable responses in association with chemotherapy and/or radiotherapy. Overall response rate (complete and partial [15]) was 100%, that is, there was complete response (CR) in 5 and partial response (PR) in 18.
In preclinical studies, T4 enhances cancer cell proliferation, migration, invasion, and angiogenesis [3, 10, 17], apparently acting via the thyroid hormone receptor on cell surface integrin αvβ3 [3, 4]. Supraphysiologic amounts of T3 are required at this receptor to stimulate tumor cell proliferation [3, 9]. The highly unusual instances of rapid tumor regression observed following exogenous l-T4 discontinuation in the case histories provided in this paper in a variety of solid tumors are unusual in non-sex-hormone-dependent tumors.

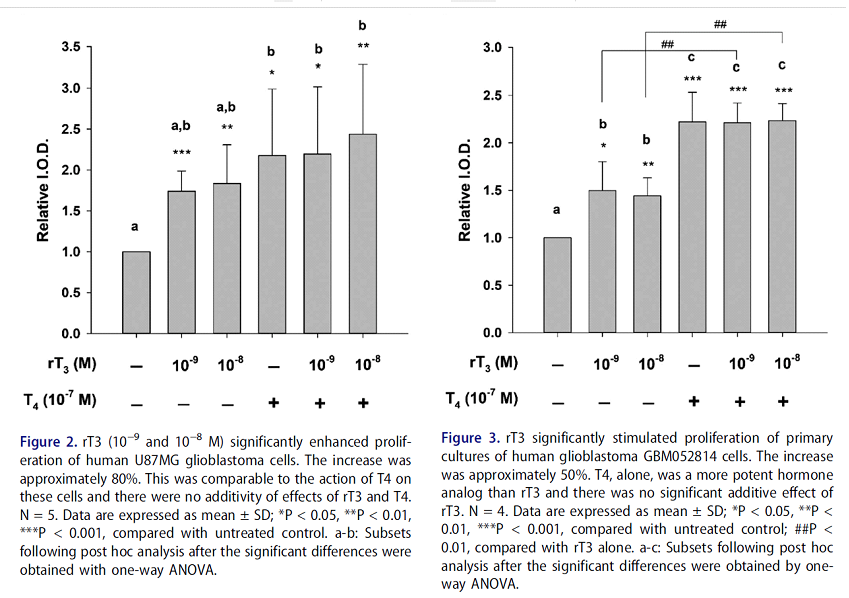
This in-vitro study shows that both T4 and rT3 enhances the proliferation of glioblastoma cells (aggressive brain cancer cells), see graphs below. Note the effect is not additive, T4 and rT3 have similar level of effect by themselves or combined.
For anyone who is interested the following review gives an extensive summary of the evidence so far: Hercbergs A. Clinical Implications and Impact of Discovery of the Thyroid Hormone Receptor on Integrin αvβ3-A Review. Front Endocrinol (Lausanne). 2019 Aug 23;10:565. doi: 10.3389/fendo.2019.00565. PMID: 31507530; PMCID: PMC6716053. https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6716053/ .
Cayrol, F, Sterle, HA, Díaz Flaqué, MC, Barreiro Arcos, ML, Cremaschi, GA. Non-genomic actions of thyroid hormones regulate the growth and angiogenesis of T cell lymphomas. Front Endocrinol. 2019;10:63. doi: 10.3389/fendo.2019.00063 . This review describes studies that reveal the role of T4 and the integrin αvβ3 receptor in cancer.
‘Integrin αvβ3 is a member of a large group of heterodimeric transmembrane receptors that regulate cell-cell and cell-extracellular matrix (ECM) interactions and enable cells to respond to their environment (10).‘
‘Interestingly this integrin is highly expressed in proliferating cells, like malignant cancer cells‘
‘It is well-known that the growth, invasiveness, and dissemination of a tumor are highly associated with angiogenesis.‘
‘the role of integrin αvβ3 as the membrane receptor for THs and how its activation induces the proliferation and survival of different types of cancer cells‘
‘In this review, we will focus on the role of integrin αvβ3 as the membrane receptor for THs and how its activation induces the proliferation and survival of different types of cancer cells.‘
‘Angiogenesis is the formation of new blood vessels from pre-existing ones. Even though it is a fundamental physiological event, in certain situations angiogenesis can also be negative; the formation of new blood vessels contributes to the progression of several pathologies and is crucial in tumor growth and metastasis. Consequently, angiogenesis is essential for the growth, spreading and infiltration of malignant cells within tissues (64).‘
‘A number of in vitro and in vivo studies have supported a role for THs in the proliferation of tumor cells (75, 77–79) and as proangiogenic factor in many types of cancer (15, 75, 76, 80)‘
‘At physiological free hormone concentrations T4 is maximally active at the S2 site on integrin αvβ3, however significantly higher than physiological levels of free T3 are required to induce proliferative activity via this receptor (5)‘
Summary: Cancerous Actions of Integrin αvβ3 Receptor
The above studies and reviews show how T4 acting at the integrin αvβ3 receptor at physiologic levels promotes and protects cancers. The evidence for this is strong, we can have a good degree of certainty in this ‘natural’ process.
We need to ask: Are T4 levels in the general population associated with cancer risk and mortality?