Polybrominated diphenyl ethers (PBDEs) are ubiquitous lipophilic bromine-based flame retardants introduced in the 1970s and used in soft furnishing and electrical equipment. They are mixed in, not chemically bonded and compose up to 30% of the material by weight. There are 209 possible PBDE structures called congeners which disrupt thyroid hormone action with effects that are species and congener specific. Hydroxylated PBDEs have a similar structure to thyroid hormone with more potent effects: –
Elimination half-lives vary from months to several years in larger animals. The manufacture of PBDEs was banned in 2004, the human burden peaked around 2010 and is expected to continue for generations. Ingestion is by diet, inhalation and dermal contact. Washington State Department of Health has produced a good summary of PBDEs: –
A PubMed search ‘PBDE Thyroid’ currently returns 333 studies. Interpretation of this research is problematic. Epidemiological studies show the human burden has changed over time, increasing rapidly during the 1980s and 1990s – a time when CFS/ME (yuppie ‘flu) and fibromyalgia became common. A confounding factor is that the move towards greater reliance on TFTs occurred at the same time.
Infants born to mothers with upper quartile serum PBDE levels have an IQ six or seven points lower than infants from mothers in the lower quartile. Subjects with high PBDE levels tend to have slightly lower T4 and higher TSH levels. Higher PBDE levels are associated with thyroid cancer. In vitro experiments elucidate multiple mechanisms by which PBDEs and OH-PBDEs influence thyroid hormone production and action. Animal experiments are difficult to interpret since effects vary between species and PBDE congeners. Animals have different ingestion routes (e.g. fur licking) and small animals have short elimination half-lives leading to different congener profiles. Cats exposed to PBDEs develop hyperthyroidism.
PBDEs and OH-PBDEs displace T4 from transthyretin (TTR), the major transport protein in the CSF. Brain PBDE levels are high, perhaps due to its high lipid content. The brain gets 80% of its T3 from type-2 deiodinase (D2: T4 -> T3) with the other 20% coming from the serum. Environmental scientists assisted by Tony Bianco found that PBDEs disrupt D2 activity in the brain: –
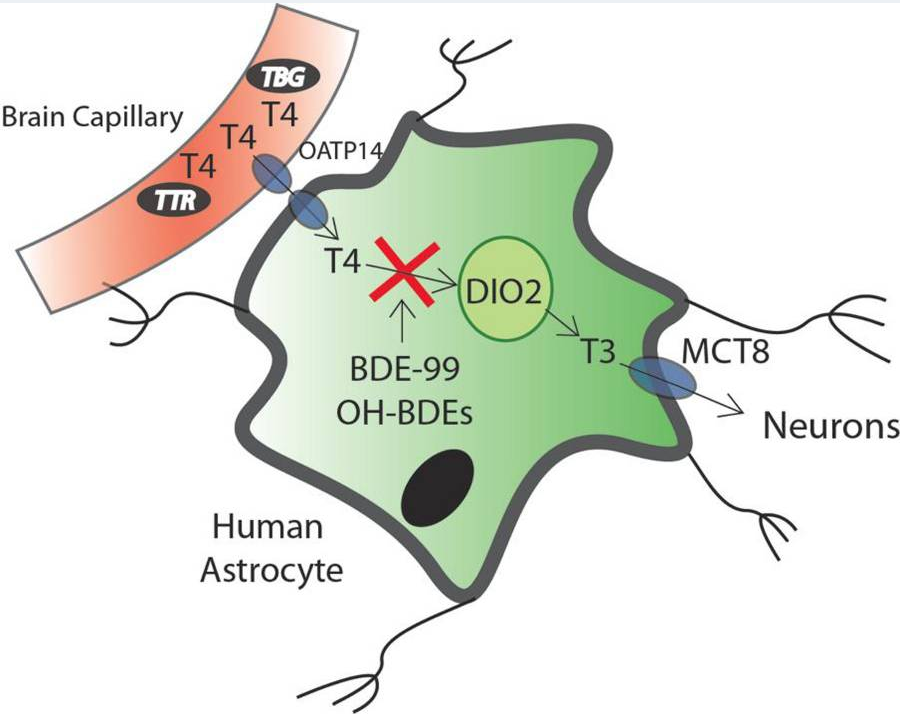
Reprinted with permission from Chem. Res. Toxicol.20152861265-1274
Publication Date:May 24, 2015
https://doi.org/10.1021/acs.chemrestox.5b00072 Copyright 2015 American Chemical Society.
A Canadian study found increased prevalence of hypothyroidism in women with high PBDE levels, the younger group having a greater increase in prevalence that the older group: –
The authors suggest this might be due to PBDE exposure during puberty in the younger group. Hypothyroidism is much more common in old age, so perhaps the fractional increase due to PBDEs will be higher in the younger group. Minor changes in hormone levels caused by PBDEs are unlikely to have a significant affect. Patients with marginal TFT results and pronounced symptoms will seek endocrine advice and perhaps be misdiagnosed as suffering from primary hypothyroidism.
PBDEs are a strong candidate for thyroid hormone disruption by multiple mechanisms. The human burden has only just started to decline and will continue for decades. There is a large body of epidemiological and in vitro evidence of thyroid hormone disruption.
My serum PBDE levels were low, I was unable to obtain a serum OH-PBDE assay. Assuming I might be susceptible to OH-PBDEs I removed potential sources from our house (an old sofa with flame retardants and bedroom carpets with foam underlay that had turned to dust). A way to eliminate PBDEs was needed.


