Pain is conducted to the brain along various pathways via receptors. Different types of pain (and other sensations such as touch) have different pathways. Receptors called “N-methyl-D-aspartate receptors” (NMDA receptors) have been shown to mediate chronic pain and visceral pain, including the pain from distending a balloon in the colon. These receptors are expressed in the spinal column and in peripheral tissues. NMDA receptors do not convey non-visceral acute pain, such as a pinch to a rat’s paw.
The following video shows how NMDA receptors have a ‘magnesium block’ that requires strong depolarization before it is removed and allows the entry of calcium into the post-synaptic neuron. When Mg levels are low the threshold for NMDA depolarization is lower and depolarization is enhanced.
NMDA receptors have multiple functions, they convey pain, signals to muscles and play a major role in memory and brain function (long term potentiation – LTP). We are primarily concerned with the first two. We will see how low intracellular Mg++ causes hyperalgesia – a lower threshold for NMDA depolarization.
NMDA receptors also play a major role in hyperalgesia via a process called “wind-up”. Wind-up occurs when repeated stimulation produces increasing sensitivity to pain.
A remarkable experiment demonstrated that the NMDA pathway was responsible for wind-up hyperalgesia in the oesophagus. Volunteers had an electrode inserted in the oesophagus about 19cm from the stomach. Their pain tolerance to electrical stimulation was measured. Another electrode affixed to the big toe was used to measure remote pain levels. Dilute hydrochloric acid was then infused into the oesophagus 3cm from the stomach. Pain thresholds were reassessed; whilst there was no change in the sensitivity of the big toe, pain thresholds in the oesophagus were lower. Wind-up had occurred, but it was local to the area of stimulation. Note however, that the inflammation from the acid did not approach the location of the electrode in the oesophagus. The researchers concluded that the wind-up occurred in the part of the spinal column receiving pain signals from the oesophagus, a phenomenon known as ‘central hyperalgesia’. The volunteers then received an injection of the NMDA receptor antagonist Ketamine. The oesophageal pain threshold returned to normal. Some volunteers received Ketamine before the experiment and did not develop hyperalgesia. This demonstrates that hyperalgesia in the gut is an NMDA receptor disorder.
In simple terms the above experiment shows that gut hyperalgesia is an NMDA receptor disorder with a central hyperalgesia component. IBS patients exhibit widespread visceral and chronic hyperalgesia suggesting a generalized NMDA receptor disorder. The induction of central hyperalgesia is important as IBS patients have central hyperalgesia which is blamed on psychological factors.
Low dose tri-cyclic antidepressants, such as Amitriptyline have been successful in the treatment of patients with IBS and have been shown to reduce rectal sensitivity. Amitriptyline is an NMDA receptor antagonist.
IBS symptoms are known to worsen during periods of physical or mental stress. In a fascinating study it was shown that physical and psychological stress lowers the threshold of rectal perception and sensation of pain, this visceral hypersensitivity was not seen in healthy controls. Acute stress altered somatic (anal) sensitivity in both patients and controls, but visceral (rectal) sensitivity was altered in IBS patients only.
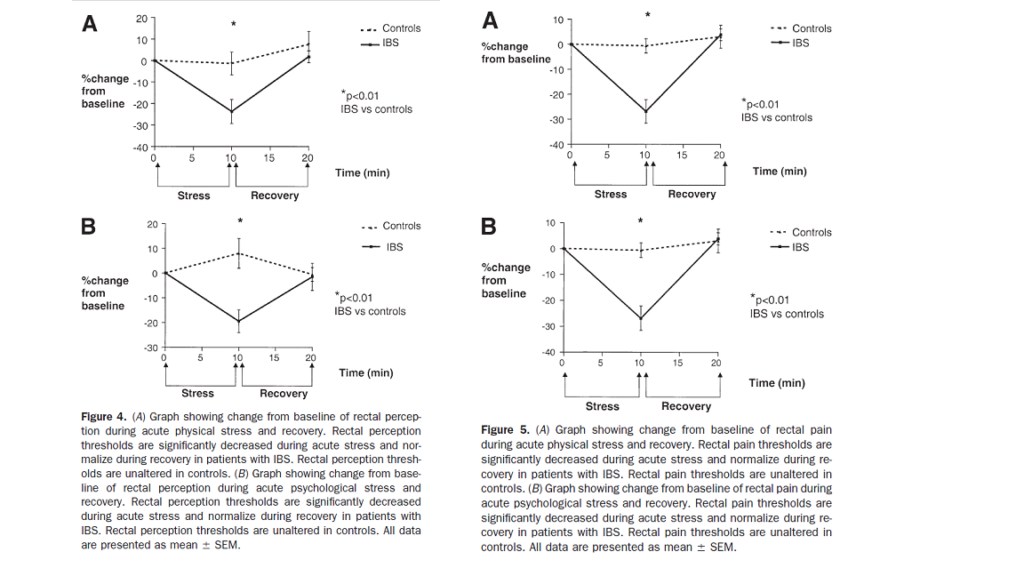
Murray CDR, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. The Effect of Acute Physical and Psychological Stress on Gut Autonomic Innervation in Irritable Bowel Syndrome. Gastroenterology 2004;127(6):1695-1703
A follow-up study demonstrated that when the patients were pre-treated with amitriptyline three quarters of them had a significant reduction in symptoms and no longer exhibited the reduction of visceral thresholds when subjected to stress.
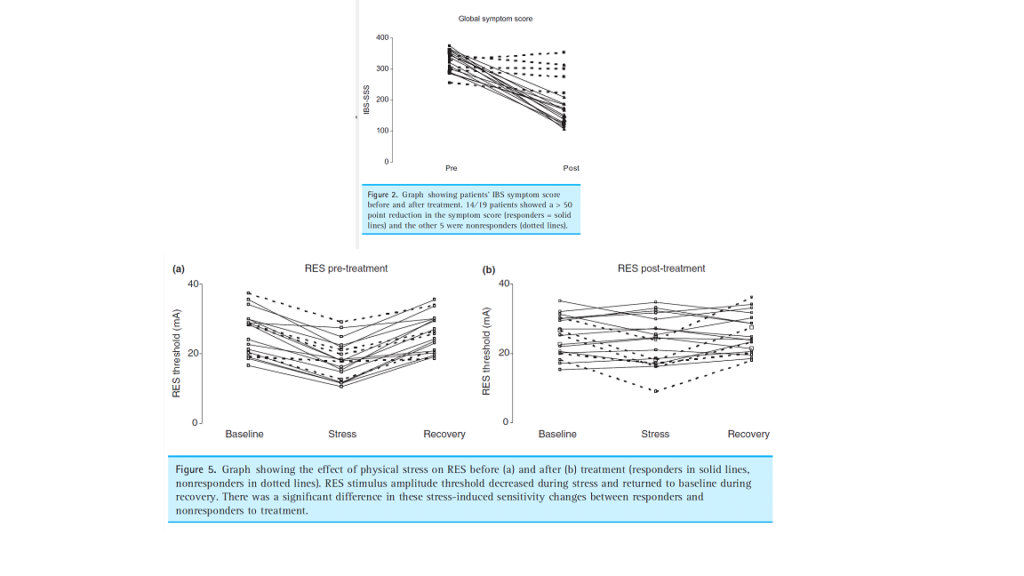
Thoua NM, Murray CD, Winchester WJ, et al. Amitriptyline modifies the visceral hypersensitivity response to acute stress in the irritable bowel syndrome.
Aliment Pharmacol Ther 2009;29(5):552–60.
The above studies show that the induction of visceral hypersensitivity by stress in IBS patients is an NMDA disorder and not a psychiatric disorder.
Next, we look at the role of the Enteric Nervous System.