Budesonide is a corticosteroid that has a potent local anti-inflammatory effect because of its high affinity for glucocorticoid receptors in the gut. It also has an ‘almost complete first-pass metabolism in the liver’ which in plain English means it doesn’t stay very long in circulation. Budesonide works on the gut and has little effect anywhere else.
A ‘full’ dose of budesonide is typically 9 mg/day with 6 mg/day (occasionally 3 mg/day) the usual maintenance dose. A randomised, double-blind, placebo-controlled study looked at the effectiveness of budesonide and what happens when treatment is discontinued.
Figure 4 shows how budesonide restores the collagen layer to its normal thickness.
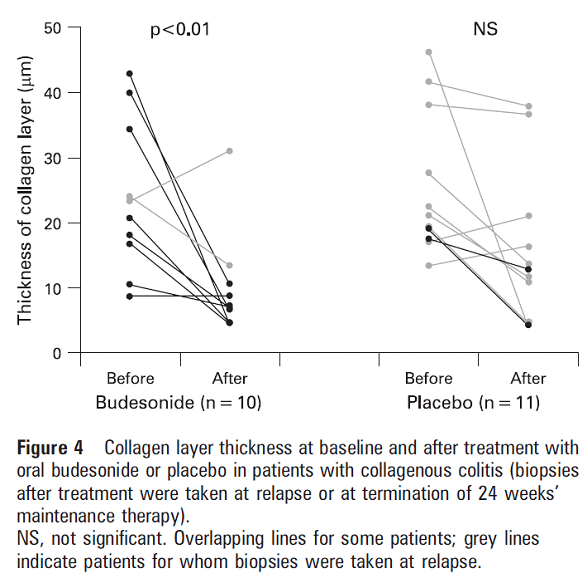
Bonderup, Ole & Hansen, J & Teglbjaerg, P & Christensen, Lisbet & Fallingborg, Jan. (2008). Long-term budesonide treatment of collagenous colitis : A randomized, double blind, placebo-controlled trial. Gut. 58. 68-72. 10.1136/gut.2008.156513.
Figure 3 shows how once budesonide treatment is stopped the rate of relapse is similar regardless of whether the patient has been on short or long-term treatment.
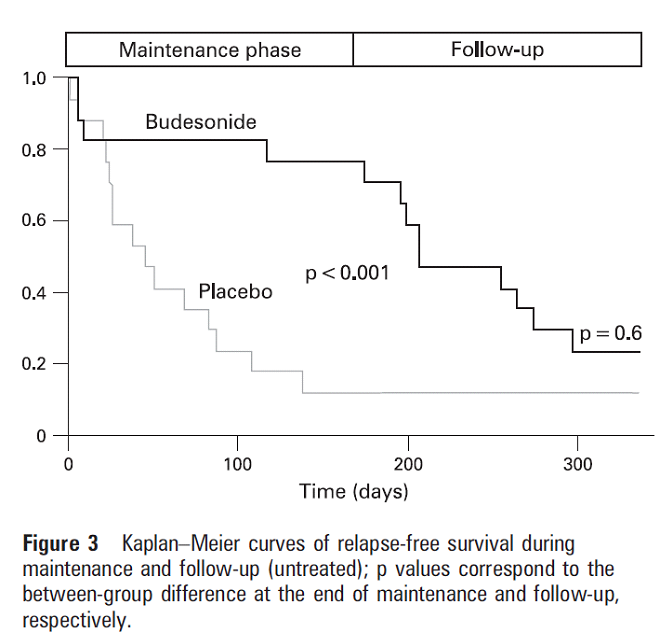
These graphs show how patients relapse after budesonide treatment is stopped. The median times to relapse were remarkably similar (39 days in maintenance treatment group, 38 days in the placebo group).
Another study showed similar results. Budesonide was effective but most patients relapsed after treatment was discontinued. The graphs are tricky to follow. Graph A shows replase rates over time during the double blind therapy. Graph B shows additional relapse after treatment was discontinued. To get an idea of what is happening compare Placebo (A) with Budesonide (B).
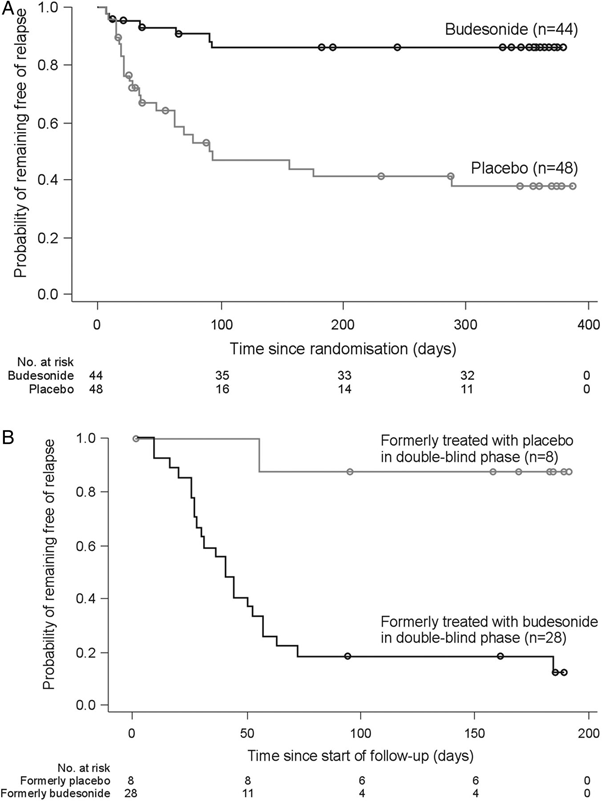
Münch A, Bohr J, Miehlke S on behalf of the BUC-63 investigators, et al. Low-dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo-controlled, 12-month trial
Gut 2016;65:47-56.
It is interesting to note that the patients in the above study had mild CC (they only required 4.5 mg budesonide a day). Nonetheless, 82.1% of these patients relapsed after this modest dose was discontinued.
These and other studies point to the presence of a noxious factor in the faecal stream.