How might D2 work?
Here we look at how TSH stimulates deiodinase. We have seen how patients with a subnormal TSH may not have an elevated TSH in the presence of low normal fT3 and fT4. Since most T3 comes from D2 (especially when fT4 is low) we can deduce D2 activity is abnormally low and consequently local T3 levels will be low.
Why is D2 Important?
Before looking at evidence we might carry out a thought experiment and design a system for regulating D2 / D3. We can achieve homeostasis in normal circumstances by having D2 and D3 respond accordingly to T4 and T3 levels giving stable T3 levels. However, in certain circumstances we want to slow down metabolism, for example, if there is severe illness or starvation. Increased D1 and reduced D2 activity will achieve this for serum T3 levels (to a degree). But how can we stop the intracellular D2 / D3 mechanism intervening and ‘correcting’ reduced intracellular T3? How can we tell the cell not to do this? Since the ‘speed up / slow down’ messenger for the thyroid is TSH it could also act as the messenger for D2 / D3 regulated cells. This is quite likely since most T3 comes from D2 activity, if increased TSH is to provide more hormone AND an increased T3 / T4 ratio D2 must respond to the TSH message (or a parallel message). This makes perfect sense; endocrinologists distrust common sense so we seek further evidence.
Expression of TSH receptors outside the thyroid
TSH Receptors are present in many tissues including the brain suggesting these tissues respond to TSH. In some tissues TSH stimulates D2. TSH also has other actions such as controlling migratory and breeding activities.
Williams, Graham H.. “Extrathyroidal expression of TSH receptor.” Annales d’endocrinologie 72 2 (2011): 68-73 .
Little is understood about these other roles of TSH but clearly a low TSH should be avoided if possible.
Expression of TSH receptors in the brain
If TSH regulates D2 activity in the brain, we would expect TSH receptors to be present. The following study concludes: these findings clearly demonstrate the expression of the TSH receptor gene in the brain in both neuronal cells and astrocytes. TSH receptors are not only present in the human brain but also located in the cells responsible for D2 activity. I struggle to follow this type of study which is beyond my expertise so can’t make any comments other than note it found TSH receptors in astrocytes.
TSH stimulates deiodinase in the thyroid
This early study is interesting because it shows that TSH stimulates deiodinase in the thyroid gland. A corollary is that patients without a functioning thyroid will not have thyroidal deiodinase and consequently it cannot be assumed that they will respond to thyroid hormone supplementation in the same way as healthy subjects.
The thyroid expresses D1 which was confirmed by the inhibiting effects of propylthiouracil (PTU) and the conversion of T3 to T2. Note: the increase in T4 -> T3 conversion is greater than that of T3 -> T2 and conversion of RT3 to T2 is also increased. The lack of effect in the liver and kidney suggests that TSH receptors are involved in this process. This study shows that TSH stimulates D1 activity in the thyroid.
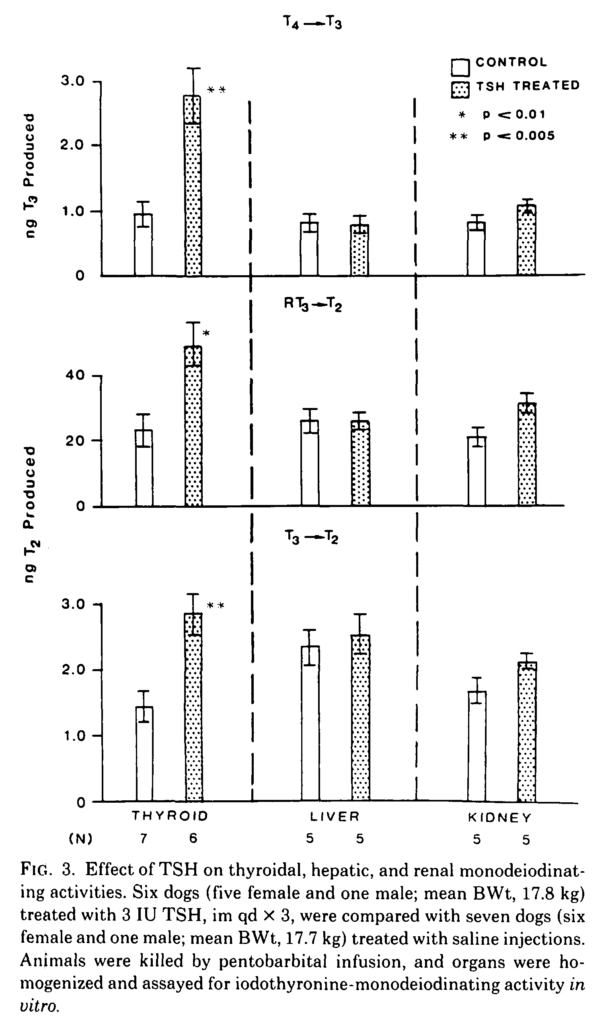
SING-YUNG WU, Thyrotropin-Mediated Induction of Thyroidal Iodothyronine Monodeiodinases in the Dog, Endocrinology, Volume 112, Issue 2, 1 February 1983, Pages 417–424, https://doi.org/10.1210/endo-112-2-417
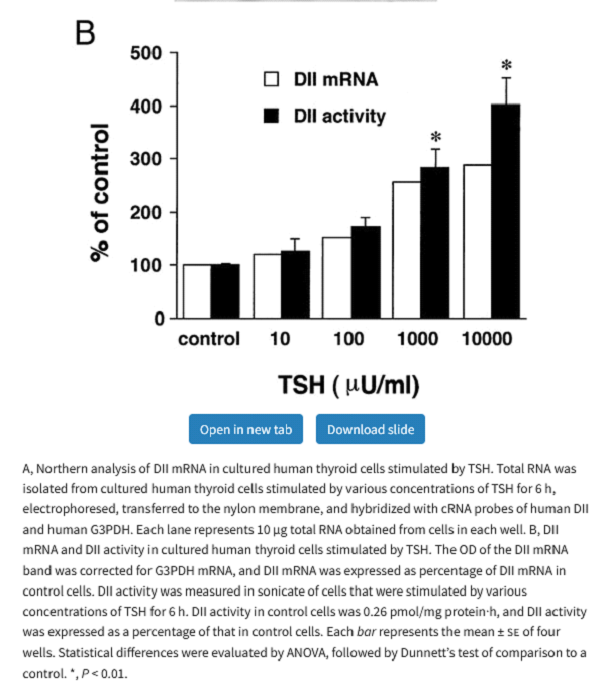
Generally, the thyroid has little D2 expression or activity but in circumstances such as a thyroid adenoma or Graves’ disease D2 expression can increase dramatically and it has been demonstrated that TSH increases D2 activity in the thyroid.
TSH stimulates D2 in brown fat
The following rather complicated study shows that TSH stimulates D2 activity in brown adipose tissue (BAT). It’s not clear how much this contributes to the overall effect of BAT on thermogenesis, but it demonstrates the principal that TSH acting through TSH receptors stimulates D2.
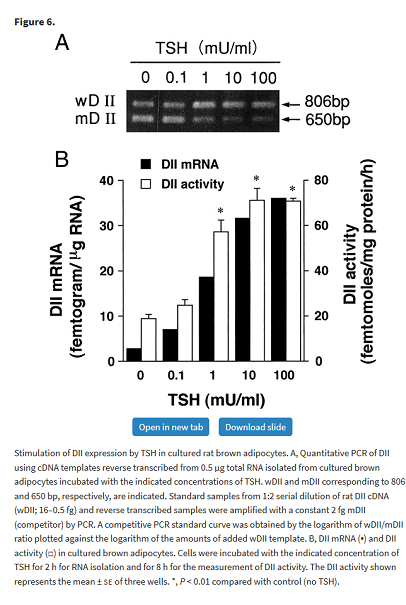
Masami Murakami, Yuji Kamiya, Tadashi Morimura, Osamu Araki, Makoto Imamura, Takayuki Ogiwara, Haruo Mizuma, Masatomo Mori, Thyrotropin Receptors in Brown Adipose Tissue: Thyrotropin Stimulates Type II Iodothyronine Deiodinase and Uncoupling Protein-1 in Brown Adipocytes, Endocrinology, Volume 142, Issue 3, 1 March 2001, Pages 1195–1201, https://doi.org/10.1210/endo.142.3.8012
TSH stimulates D2 in human osteoblast
Osteoblasts are responsible for bone formation. The black bars in graphs B and D below show that TSH regulates D2 activity in a dose dependant manner in the two cell types (SaOS-2 and NHOst) that express TSH receptors.
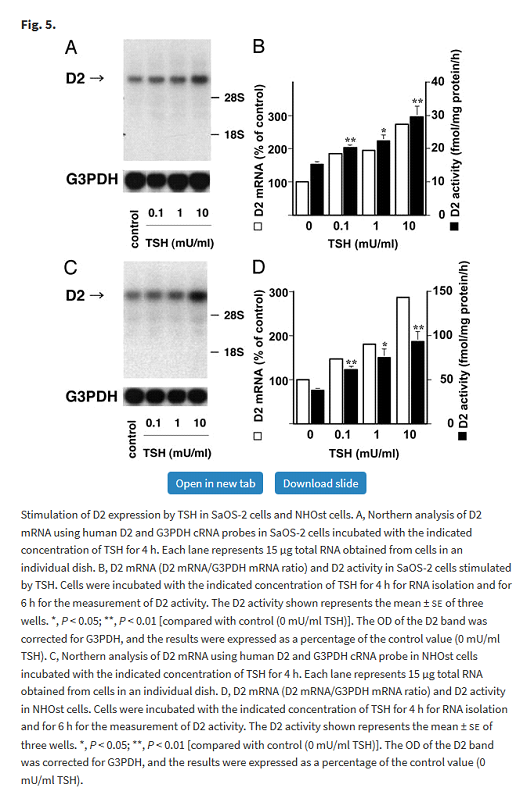
Tadashi Morimura, Katsuhiko Tsunekawa, Takayuki Kasahara, Koji Seki, Takayuki Ogiwara, Masatomo Mori, Masami Murakami, Expression of Type 2 Iodothyronine Deiodinase in Human Osteoblast Is Stimulated by Thyrotropin, Endocrinology, Volume 146, Issue 4, 1 April 2005, Pages 2077–2084, https://doi.org/10.1210/en.2004-1432
The authors commented:
In short, TSH stimulates D2 activity and thyroid hormones suppress D2 activity. In the case of a subnormal TSH this delicate balance will be disrupted with reduced bone formation. Subnormal TSH has potential implications for bone health.
An interesting feature of this study is that it found that whilst T3 and T4 reduced D2 activity, reverse T3 (rT3) which is assumed to be inactive also suppressed D2 activity. See Reverse T3 Inhibits T4 to T3 Conversion – An Enigma for more information.
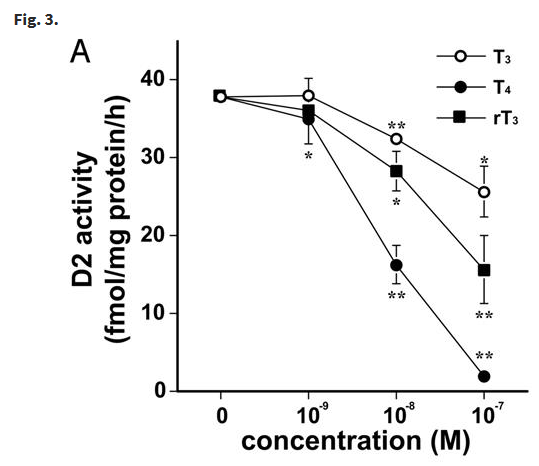
Tadashi Morimura, Katsuhiko Tsunekawa, Takayuki Kasahara, Koji Seki, Takayuki Ogiwara, Masatomo Mori, Masami Murakami, Expression of Type 2 Iodothyronine Deiodinase in Human Osteoblast Is Stimulated by Thyrotropin, Endocrinology, Volume 146, Issue 4, 1 April 2005, Pages 2077–2084, https://doi.org/10.1210/en.2004-1432
Evidence for cAMP-independent thyrotropin (TSH) effects on astroglial cells
Earlier we noted that astrocytes, a type of glial cell in the brain, expresses TSH receptors and D2 activity. This study shows how TSH has a profound effect on D2 activity in these cells.
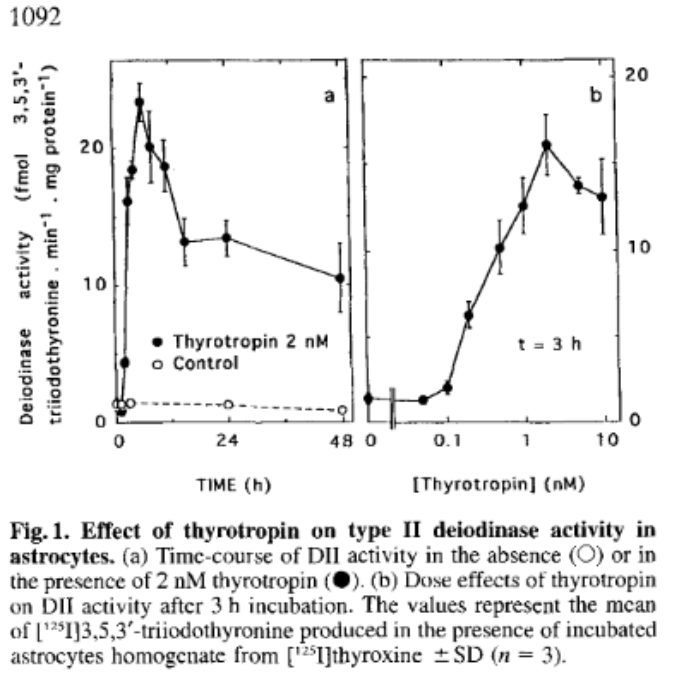
Saunier B, Pierre M, Jacquemin C, Courtin F (1993) Evidence for cAMP-independent thyrotropin effects on astroglial cells. Eur J Biochem 218: 1091–1094. (doi:10.1111/j.1432-1033.1993.tb18469.x).
The authors observed that stimulation by TSH of hormone production and D2 in the astrocytes regulates brain T3 levels. Thus, subnormal TSH secretion will reduce available hormone and D2 conversion of T4 to T3 in the brain leading to brain hypothyroidism to a degree which is not reflected in blood tests. Restoring normal serum hormone levels will not restore brain euthyroidism because the resultant reduction in TSH will further reduce brain D2 activity and hence brain T3.
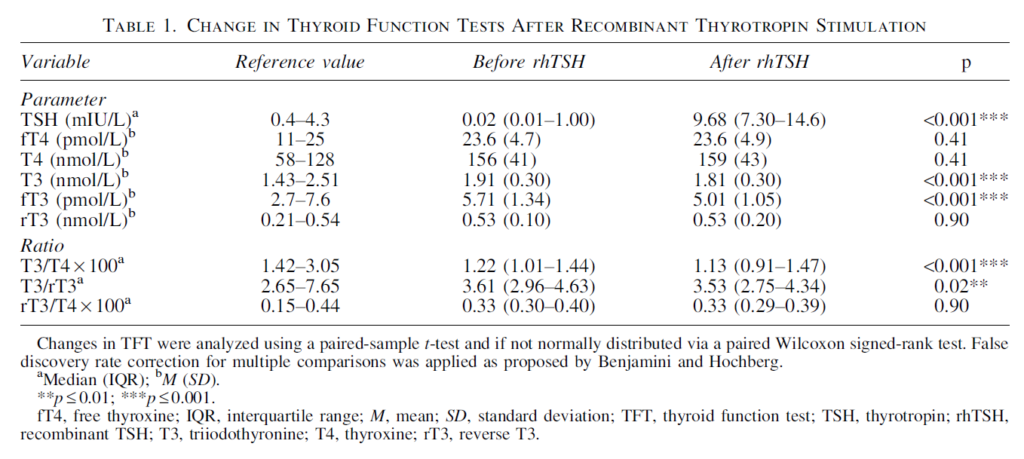
Effects of Thyrotropin on Peripheral Thyroid Hormone Metabolism and Serum Lipids
Ideally, we would carry out a study in healthy humans measuring their T3 and fT3 levels whilst delivering variable levels of TSH. This study is such an attempt which surprisingly found that T3 and fT3 was reduced by increased TSH levels.
Unfortunately, the study did not use thyrotropin (TSH), it used ‘human recombinant TSH (rhTSH)’ instead. Although this is described as ‘identical to human TSH’ it doesn’t seem correct. As noted earlier TSH has many isoforms with varying bioactivity – both thyroid stimulating bioactivity and deiodinase stimulating bioactivity. TSH bioactivity increases with increasing TRH stimulation of the thyrotroph. Thus, rhTSH has considerable differences to natural human TSH. Which isoform does it mimic? rhTSH was developed to provide a means of stimulating thyroidal secretion, to the best of my knowledge it’s deiodinase stimulating properties are unknown. Thyroid hormone levels were measured three days after rhTSH injection raising the possibility that more rapid effects have been missed. Another concern is that the selected patient group have supra-physiological fT4 levels which will act to suppress D2 activity. Nonetheless this study shows that we do not have a detailed understanding of how TSH stimulates deiodinase.
Thyroid. 2018 Feb;28(2):168-174. doi: 10.1089/thy.2017.0330. Epub 2018 Feb 1.
Effects of Thyrotropin on Peripheral Thyroid Hormone Metabolism and Serum Lipids.
Beukhof CM1, Massolt ET1, Visser TJ1, Korevaar TIM1, Medici M1, de Herder WW1, Roeters van Lennep JE2, Mulder MT2, de Rijke YB1,3, Reiners C4, Verburg FA4,5, Peeters RP1.
Conclusion
There is extensive evidence (but not formal proof) that TSH stimulates D2 activity. TSH has varying bioactivity which is not reflected in TSH assays. This, along with considerable diurnal and menstrual fluctuations means that over-reliance should not be placed on TSH levels. TSH resulting from reduced TRH stimulation has reduced bioactivity which further complicates the interpretation of TSH assays.
Experimental evidence and blood test results indicate that bioactive TSH regulates D2 activity. D2 regulates intracellular T3 in vital organs such as the brain, skeletal muscle and heart. Thus, serum T3 and TSH do not accurately reflect intracellular T3 or thyroid hormone signalling. Blood tests do not reflect thyroid status. They give an indication which combined with skilful interpretation and clinical presentation can be useful for diagnosis and therapy.
Research into subnormal TSH secretion should include : –
- A study of the effects of TRH supplementation in patients without a thyroid on levothyroxine monotherapy. Measuring TSH, fT3 and fT4 will show the relationship between TSH and peripheral deiodinase.
- A placebo-controlled trail of TRH supplementation in symptomatic patients with a subnormal TSH. TRH might restore normal TSH secretion and bioactivity.
- A placebo-controlled study of L-T3 therapy in symptomatic patients with a subnormal TSH in order to determine what dose of L-T3 is clinically effective.
Let’s look at a real case of subnormal TSH secretion.


